
Frozen testicular tissue can still make sperm 20 years later, according to scientists who say it can help young cancer patients to later have children.
Male testis tissue that is cryopreserved can be reimplanted after more than 20 years and will go on to make viable sperm, according to a new study in rodents in the open-access journal PLOS Biology by Eoin Whelan of the School of Veterinary Medicine at the University of Pennsylvania.
But the work together with colleagues that was published on May 10 found that the long delay comes with a cost in reduced fertility compared to tissue that is only briefly frozen.
The results may have important implications for the treatment of boys with cancer, for whom chemotherapy may be preceded by harvesting and freezing of testicular tissue for eventual reimplantation.
The rate of survival for childhood cancers has increased dramatically in the last several decades, but a serious side effect of treatment is diminished fertility later in life. A potential treatment would be to harvest, freeze, and reimplant testicular tissue, which contains stem cells, a procedure which has recently been shown in a macaque model to restore fertility, at least after short-term freezing.
But for pre-pubertal boys with cancer, reimplantation may not be feasible for a decade or more after harvesting, raising the question of how long frozen spermatogenic stem cells (SSCs) can remain viable.
To explore this question, the authors thawed rat SSCs that had been cryopreserved in their laboratory for more than 23 years, and implanted them in so-called nude mice, which lack an immune response that would otherwise reject the foreign tissue.
They compared the ability of the long-frozen SSCs to generate viable sperm to SSCs frozen for only a few months, and to freshly harvested SSCs, all from a single rat colony maintained over several decades.
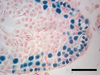
The authors found that the long-frozen SSCs were able to colonize the mouse testis and generate all of the necessary cell types for successful sperm production, but not as robustly as SSCs from either of the more recently harvested tissue samples. While the long-frozen SSCs had similar profiles of gene expression changes compared to the other samples, they made fewer elongating spermatids, which go on to form swimming sperm.
These results have several important implications.
First, they point out the importance of in situ testing of SSC viability, rather than relying on biochemical or cellular biomarkers, in determining the potential of cryopreserved cells, which may not reflect the actual loss of stem cell potential over time. Second, while there currently are no protocols that can expand human SSCs for reimplantation—a requirement for clinical development of this treatment—such protocols may need to consider time-dependent degradation of viability, assuming human SSCs mimic those of rats. Finally, and this is the good news, viability is by no means lost during long-term cryopreservation, suggesting that it may be possible to identify and mitigate the key drivers of loss of viability, in order to improve the reproductive options of boys whose childhood cancers are successfully treated.

Whelan adds, “Our study showed that rat spermatogonial stem cells can be successfully frozen for over 20 years, transplanted into an infertile recipient animal and regenerate the ability to produce sperm, albeit at a reduced rate. This could provide a method to recover the loss of fertility in prepubertal boys treated for cancer.”
The freely available paper in PLOS Biology: http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.300…
Recommended from our partners
The post Fertilitiy Hope: Frozen Testicular Tissue Still Viable After Two Decades appeared first on Zenger News.